Our Products
We have extensive knowledge of producing and supplying Active Pharmaceutical Ingredients to customers around the world, in accordance with the highest standards and regulations.
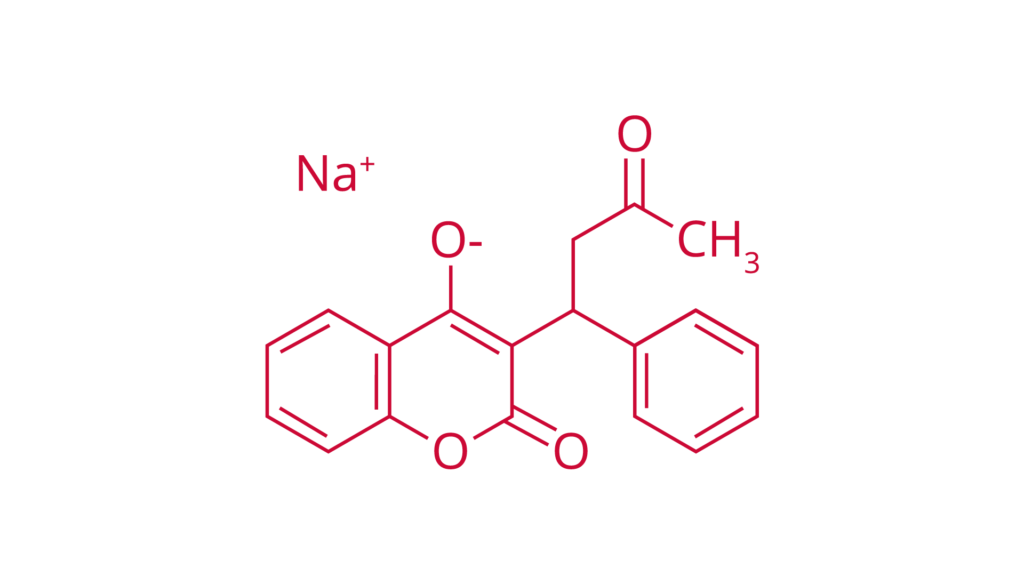
Warfarin sodium
Ph. Eur.
CAS 129-06-6
Warfarin products are used as anticoagulants for multiple indications.
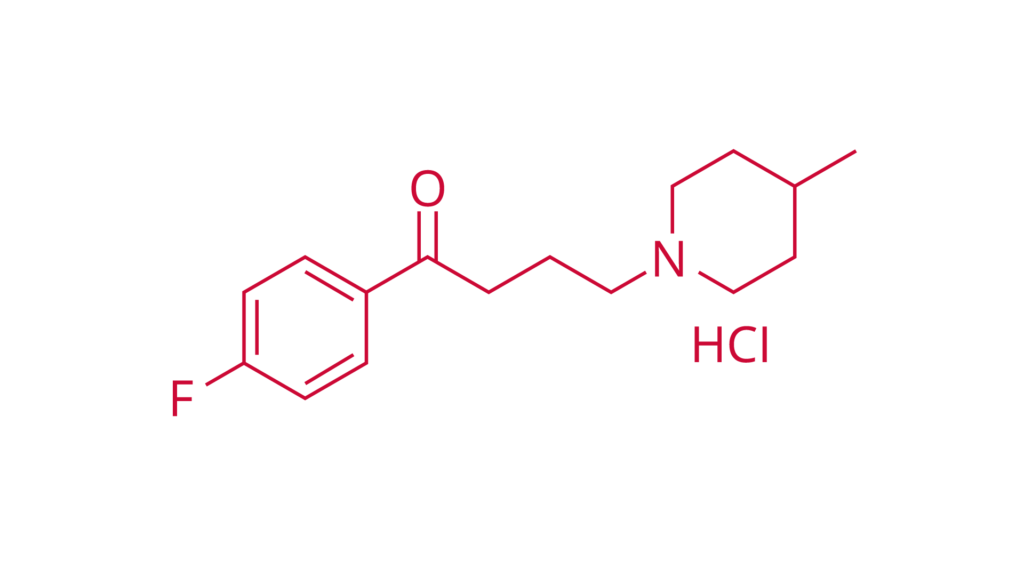
Melperone hydrochloride
E-DMF
CAS 1622-79-3
Melperone hydrochloride is an antipsychotic agent.
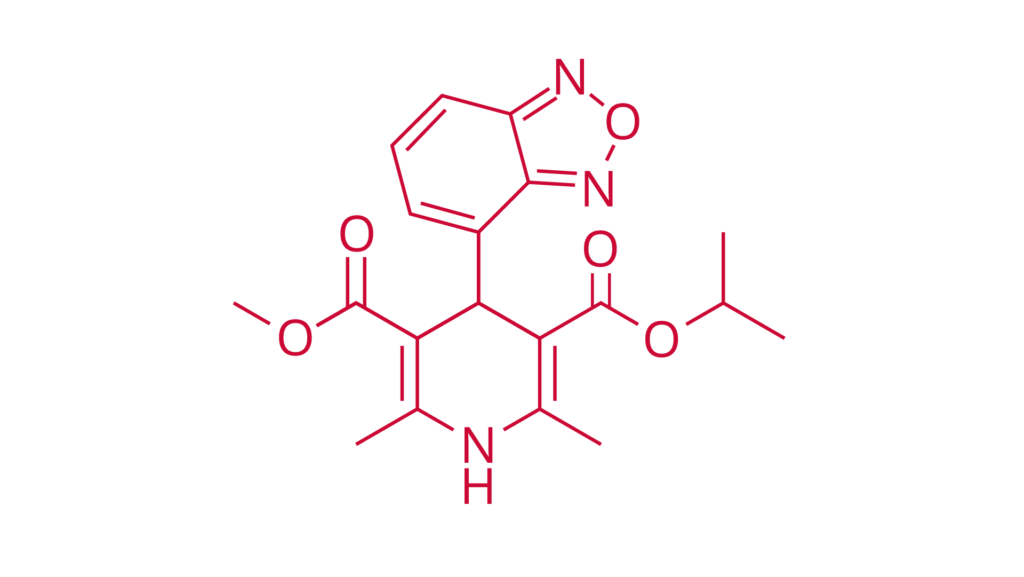
Isradipine
Ph. Eur., USP
CAS 75695-93-1
Isradipine is a calcium channel blocker used to treat hypertension.
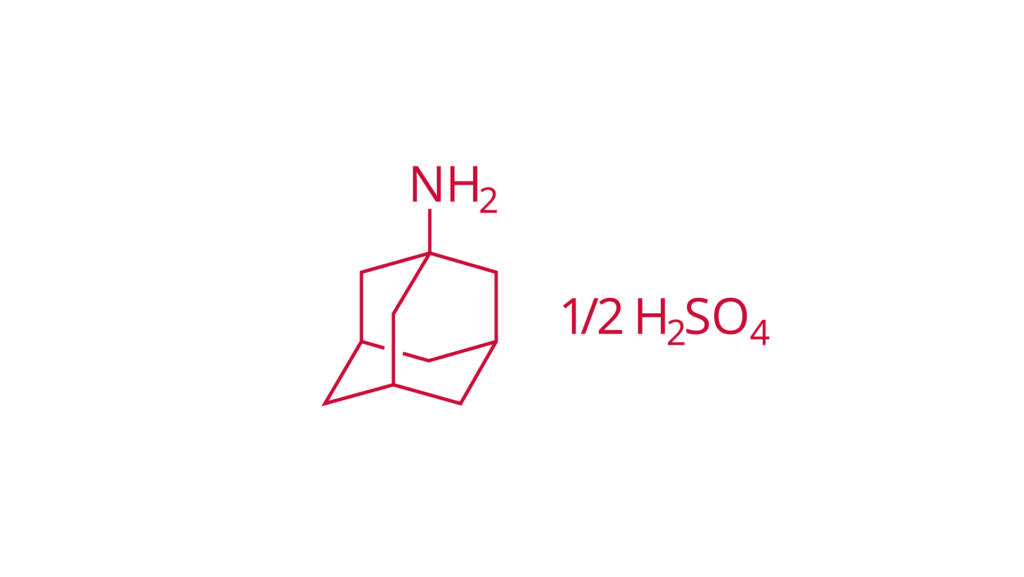
Amantadine sulfate
DAC
CAS 31377-23-8
Amantadine sulfate is used in the treatment of central nervous system disorders.
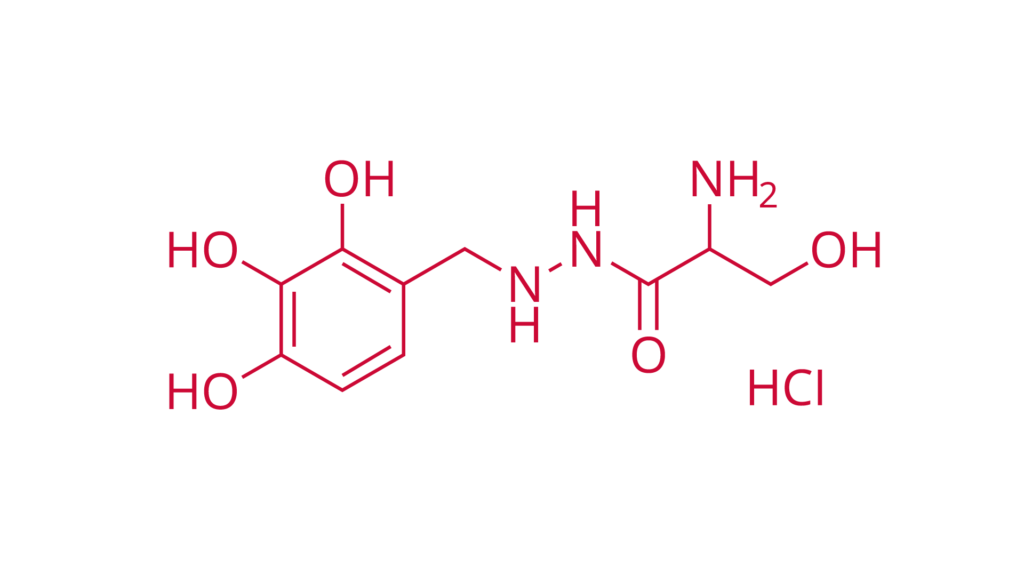
Benserazide hydrochloride
Ph. Eur.
CAS 14919-77-8
Benserazide hydrochloride is used in combination with L-Dopa in the treatment of Parkinson’s disease.
Order now
Send a request in the form below and we’ll get back to you with a quote.
[formidable id=1]
